Bloodborne Pathogens (BBP)
In 1993, CAL/OSHA published the Bloodborne Pathogens Rule (Title 8 CCR GISO 5193); the fundamental premise of this rule is an approach to infection control termed Universal Precautions.
![]() Important Information
Important Information
Universal Precautions
Universal Precautions assumes that all human cells, cell lines, human blood, blood products, and certain body fluids are contaminated with HIV, HBV, HCV, or other bloodborne pathogens and that these materials be handled accordingly.
The Bloodborne Pathogens Standard (29 CFR, Bloodborne Pathogens. – 1910.1030) applies to all occupational exposure to blood or other potentially infectious materials. Blood means human blood, human blood components, and products made from human blood. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and can cause disease in humans. These pathogens include, but are not limited to, hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Additionally, “Other Potentially Infectious Materials” (OPIM) are included under this standard. OPIM means (1) The following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; (2) any unfixed tissue or organ, including cells and cell lines, (other than intact skin), from a human (living or dead); and (3) HIV-containing cell or tissue cultures, organ cultures, and HIV, HBC, or HCV (or other) containing culture medium or other solutions, and blood, organs, or other tissues from experimental animals infected with HIV, HBV or HCV. The above additionally applies to non-human primate materials.
In accordance with the above information, BBP training (considered a Tier II level training) is mandatory and is available under Bloodborne Pathogens (EHS—1600—PROG). This course is entirely web based and requires annual updates (EHS—1601—PROG), also available on the web. To help determine if a worker is at risk for contact with BBP, please use the questions listed below:
![]() Important Information
Important Information
Is Bloodborne Pathogen training required? If a single box can be checked as yes, BBP training is required.
Will the person:
- Work with human blood, blood products or body fluids?
- Work with unfixed human cells (including tissue culture cells and cell lines), human tissues or organs?
- Work with non-human primates (NHP) or NHP blood, blood products or body fluids?
- Work with unfixed NHP cells (including tissue culture cells and cell lines), NHP tissues or organs?
- Work with bloodborne pathogens (e.g. HIV, HBV, HCV or other infectious agents able to be spread via blood)?
- Work with animals or animal tissues that have been infected with a BBP?
- Perform tasks which may potentially result in exposure to human or animal blood, body fluids, organs, or tissues which are infected with the hepatitis B virus or other bloodborne pathogens?
- Handle sharp instruments such as knives, needles, scalpels, or scissors which have been used by others working with human blood or other potentially infectious materials to include human organs, tissue or body fluids or used by others working with similar body parts and fluids from animals infected with the hepatitis B virus or other bloodborne pathogens?
If the answer to any of the above questions is yes, then the worker is considered to be at occupational risk of contracting Hepatitis B or other bloodborne pathogens. All workers at risk must take the Bloodborne Pathogen Training. Registration and completion of the appropriate courses are required within the first month of work at Stanford University. Supervisors or PIs who oversee workers that are required to take Bloodborne Pathogens training are themselves required to take Bloodborne Pathogens training even if they will not be potentially exposed to bloodborne pathogens.
![]() Important Information
Important Information
BBP is…
BBP course + Annual Refresher = BBP Training
Aerosol Transmissible Diseases (ATD)
The Stanford University Aerosol Transmissible Disease Institutional Exposure Control Plan (ATD ECD) is designed to comply with the California OSHA Aerosol Transmissible Disease Standard (Title 8, Section 5199 and Section 5199.1). The ATD ECP addresses issues related to the elimination, minimization and protection of Stanford University personnel to airborne transmissible diseases from both humans and animals (zoonotic diseases). Principal Investigators (PIs) and supervisors should refer to the ATD ECP as a resource for exposure control background, issues and regulatory procedures.
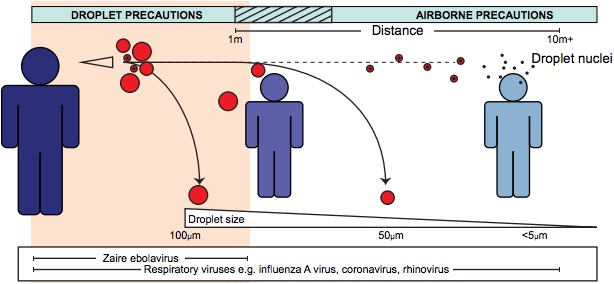
Credit: Ian M. Mackay PHD
![]() Important Information
Important Information
Droplet vs. Airborne
Droplet spread and airborne transmission are different in a very important way:
Droplet spread organisms can only contaminate nearby air while airborne organisms can contaminate over a much wider area. (Figure 1)
Research Laboratories
The Stanford University ATD ECP, in conjunction with this Stanford University Biosafety Manual, serves as the basic ATD Biosafety Plan for Laboratories.
The ATD requires laboratories to adopt standard biosafety practices to protect laboratory workers when handling materials containing pathogens that may be spread through aerosols and which can cause serious disease. The Stanford University Institutional Biosafety Committee includes, as part of its charge and following the Biosafety in Microbiological and Biomedical Laboratories (BMBL) (https://www.dir.ca.gov/title8/5199.html), oversight of issues covered within the California OSHA Aerosol Transmissible Disease (ATD) standard. As such, approved IBC protocols may detail specific requirements that must be followed in addition to the ATD ECP.
PIs and researchers should review the ATD ECP and their IBC protocol annually or when changes are made (new personnel, agents, SOPs, etc.). New personnel should be trained by the PI or their designee on both documents. All personnel covered by the ATD ECP must take EHS-1090 Aerosol Transmissible Diseases training annual.
Stanford Referring Employers: Stanford University Occupational Health Center, Department of Public Safety, and Land, Buildings and Real Estate
The Stanford ATD ECP covers “referring employers”–the Stanford University Occupational Health Center (SUOHC), Department of Public Safety (DPS), and Land, Buildings and Real Estate (LBRE) and serves as the Biosafety Plan for these units. It addresses how to eliminate or minimize exposure to materials containing pathogens that may be spread through aerosols and which can cause serious disease and addresses health and safety issues specific to the jobs and procedures being used by personnel.
An ATD Administrator is designated by each of these units and is responsible for the establishment, implementation, and maintenance of written procedures to control ATDs. The Stanford ATD ECP includes the role designated as ATD Administrator and contact information for this role.
ATD Zoonotic
The Stanford ATD ECP details work operations that require additional oversight under Section 5199.1, Aerosol Transmissible Diseases – Zoonotic.
Note on Laboratory Operations: Laboratory operations that involve samples, cultures, or other materials potentially containing zoonotic ATPs only need to comply with the overall ATD ECP unless they meet any of the three conditions outlined below.
Note on Vertebrate Animal Research Facilities: Vertebrate animal research facilities only need to perform and document a risk assessment and adopt control measures consistent with the CDC’s Biosafety in Microbiological and Biomedical Laboratories and comply with the overall ATD ECP, including required recordkeeping for zoonotic operations, unless they meet any of the three conditions outlined below.
Work requiring additional oversight include:
- Work operations that involve:
- Capturing or sampling of wildlife for the purpose of determining whether they are infected with zoonotic ATPs, or
- Collecting and disposing of wildlife for which an alert regarding the potential of zoonotic ATP infection has been issued by the CDC, CDFA, CDFG, CDPH, USDA, or USDOI, and the alert is applicable to the employer’s operations based on conditions specified in the alert, e.g., the geographic area and the species or type of animal.
- Work operations that involve establishments or operations for which the USDA or CDFA have issued a quarantine order, movement restriction, or other infection control order due to an increased risk of zoonotic ATP infection.
- Work operations that involve:
- Handling, culling, transporting, killing, eradicating, or disposing of animals infected with zoonotic ATPs as defined in subsection (a)(4), or
- Cleaning or disinfecting areas used, or previously used, to contain such animals or their wastes.
![]() Important Information
Important Information
If you perform work that you believe meets these work operations AND meets the definitions with the ATD ECP for the relevant terms (e.g., zoonotic ATPs, etc.), contact the Biosafety Officer at biosafety-officer@lists.stanford.edu prior to beginning any work.
A Biosafety Plan specific to the work will be needed, and additional oversight and approvals, such as from the IBC or other research compliance offices, may also be required.
![]() Important Information
Important Information
PI Responsibilities
According to the NIH Office of Biotechnology Activities (https://osp.od.nih.gov/wp-content/uploads/Investigator_Brochure_Recombinant_DNA_2021.pdf), the PI is responsible for the following:
- Be adequately trained in good microbiological techniques
- Provide laboratory research staff with protocols describing potential biohazards and necessary precautions
- Instruct and train laboratory staff in: (i) the practices and techniques required to ensure safety, and (ii) the procedures for dealing with accidents
- Inform the laboratory staff of the reasons and provisions for any precautionary medical practices advised or requested (e.g. vaccinations or serum collection)
- Supervise laboratory staff to ensure that the required safety practices and techniques are employed
- Correct work errors and conditions that may result in the release of recombinant DNA or synthetic nucleic acid (sNA) materials
- Ensure integrity of physical and biological containment
- Comply with permit and shipping requirements for recombinant or synthetic nucleic acid molecules
- Adhere to APB-approved emergency plans for handling accidental spills and personnel contamination
Required Training for Laboratory Workers
These courses are designed to ensure compliance with applicable external regulatory requirements.
| Course Title & STARS Number | Must Be Taken By All Who | Notes on Taking the Course |
|---|---|---|
| General Safety, Injury Prevention (IIPP) and Emergency Preparedness EHS-4200-WEB |
…work at Stanford University | Register and launch through https://axess.stanford.edu |
| Life Sciences Research Lab Safety EHS-PROG-4875 |
…work in life sciences research laboratories in the SOM, as well as others working with biological agents, hazardous chemicals & compressed gases** | Register and launch through https://axess.stanford.edu |
| Biosafety EHS-1500-WEB |
…work with biological agents** | Register and launch through https://axess.stanford.edu |
| Chemical Safety for Laboratories EHS-1900-WEB |
…work with chemicals** | Register and launch through https://axess.stanford.edu |
| Compressed Gas Safety EHS-2200-WEB |
…work with compressed gas cylinders** | Register and launch through https://axess.stanford.edu |
| Laboratory Ergonomics EHS-4800 |
…perform repetitive tasks such as microscope use, pipetting, and miscellaneous hand tool use | This is a classroom course. Register and launch through https://axess.stanford.edu |
| Computer Workstation Ergonomics EHS-3400-WEB |
…use a computer routinely | Register and launch through https://axess.stanford.edu |
| Bloodborne Pathogens EHS-PROG-1600 EHS-PROG-1601 (Recert) |
…work with human and/or non-human primate blood, blood products, cells (including tissue culture) or other potentially infectious material | BBP training must be taken annually. Register and launch through https://axess.stanford.edu Everyone who must take BBP must also create and/or update an Exposure Control Plan annually. |
| Radiation Safety Training EHS-5250-WEB |
…have never worked with radioactive materials before (also take EHS-5251 Hands-on Training) | Register and launch through https://axess.stanford.edu |
| Laser Safety EHS-PROG-4820 EHS-PROG-4821 (Recert) |
…work with Class 3 or 4 lasers | Must be taken every 3 years. Register and launch through https://axess.stanford.edu |
| DOT: Excepted Quantities EHS-PROG-2650 EHS-PROG-2651 (Recert) |
…who are required to identify, package and air ship small quantities of hazardous chemical materials | Must be taken every two years. Register and launch through https://axess.stanford.edu |
| DOT: Shipping Dangerous Biological Goods or Dry Ice EHS-PROG-2700 EHS-PROG-2701 (Recert) |
…package and/or ship dangerous biological materials, or who package and/or ship any packages containing DRY ICE | Must be taken every two years. Register and launch through https://axess.stanford.edu |
| Cryogenic and Dry Ice Safety EHS-2480 |
…work with cryogenic liquids and/or dry ice | This is a classroom course. To register, call 723-0448 |
| Orientation for Lab Safety Coordinators EHS-5200 |
…who are assigned safety/compliance tasks by their PI or lab supervisor | This is a classroom course. To register, call 723-0448 |
| Controlled Substances EHS-2125-WEB |
…work with controlled substances | Register and launch through https://axess.stanford.edu |
| Fire Extinguisher Training EHS-3825 (classroom) EHS-3850-WEB |
…work at Stanford University | To register for classroom, call 723-0448. Register and launch through https://axess.stanford.edu |
| Other courses as necessary | This is not a complete list of safety training courses that you may be required to take | Please reach out to EH&S Research Safety for help identifying the safety training that is required for your type of work. |
