Radioactivity is the spontaneous emission of charged particles or photons by an atomic nucleus that is in an unstable configuration. This event is called a nuclear transformation, a decay, or a disintegration. Each decay event involves loss of mass or charge. There are a variety of radioactive decay modes. One of the best sources of information on decay modes is the Chart of Nuclides published by the Knolls Atomic Power Laboratory. Decay schemes for selected isotopes also appear in many texts and reference books. For a more complete introductory discussion, see Alpen ch. 3, Hendee ch. 3, Turner ch. 3, or Bushberg ch. 14.
Alpha
The alpha particle is simply a helium nucleus, comprised of two protons and two neutrons. It is associated with the radioactive decay of elements of high atomic number. For example,
![]()
Each alpha particle has a charge of +2 and a mass of 4. Most have an initial kinetic energy of about 5 MeV. They are frequently accompanied by high energy gamma rays. Almost all radionuclides that decay by alpha emission have atomic number greater than 83 (bismuth). See Krane ch. 8.
Properties of α‐ particles
Because of their +2 charge and relatively low velocity, alpha particles are densely ionizing, depositing an enormous amount of energy at each collision with an attenuating atom. Thus, they lose all of their kinetic, ionizing energy after travelling a very short distance in any medium. A thin piece of paper, or the layer of dead cells on your skin surface, will completely attenuate a beam of alpha particles. Therefore, alpha particles pose no external hazard. However, if ingested, they can deliver a very large radiation dose to tissue. For example, radium is in the same column of the periodic table of elements as calcium, and is a bone seeker. Ingestion of radium can cause a very large radiation dose to blood‐ forming cells.
Beta
The beta particle is an electron that has been ejected from a neutron‐rich nucleus. It differs from an electron only because it is a product of radioactive decay. This leads us to observe that the neutron is essentially a proton with an attached electron. During the radioactive decay event, the neutron reverts to a proton, an energetic electron and a neutrino that escapes the nucleus. For example,
![]()
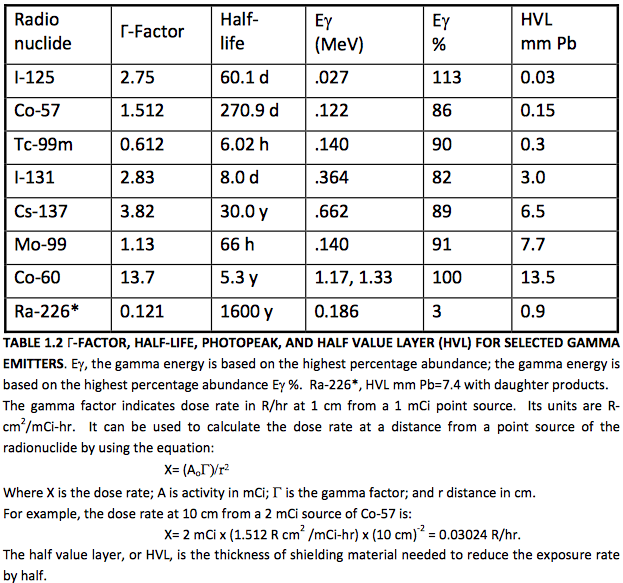
The maximum kinetic energy of the beta particle, in this example 0.156 MeV, can range from as low as 0.019 MeV for a 3H decay to as high as 1.7 MeV for a 32P decay, or 3.3 MeV for a 214Bi decay. The higher energy particles are more penetrating. See Table 1.1 for other examples of beta emitters.
Unlike the discrete energies observed for alpha particles and gamma rays, the average kinetic energy of all beta particles from a given isotopic sample is about one‐third the maximum energy that is possible for that isotope. See Figure 1.1. The maximum and average are characteristic for the isotope. For a low energy beta particle, we might ask where the missing energy has gone. To explain this, Pauli postulated the existence of a new particle, the neutrino (ν), emitted simultaneously and sharing the energy of the decay event with the beta particle. Neutrinos have little mass and no charge, and do not frequently interact with matter.
Properties of β‐ particles
As with alpha particles, beta particles are completely attenuated by small thicknesses of common materials. SeeFigure 1.2. They pose an external source of radiation dose to the skin and eyes. A beta emitter can also cause radiation dose if ingested.
A low atomic number material such as plastic is used for shielding a beta emitter. The dose rate from a point beta source with energy greater than 0.5 MeV is:
![]()
Where X is the dose rate measured in rad/hr, A is activity in Ci, and r is distance in cm. For example, the beta dose rate at 3 cm from a 1 mCi vial of P‐32 is:
![]()
Positron
A few isotopes, such as 11C, 13N, and 18F, decay by positron emission. A positron, the anti-particle of a beta particle, is emitted by a proton-rich nucleus. It has the same mass as an electron, but carries a positive charge. During the decay event a proton converts to a neutron and a positive electron, or positron, which is ejected from the nucleus. The range and specific energy loss of positrons is about the same as that of negative beta particles, but they are different in that they annihilate with an electron from the absorbing material at the end of their track, yielding two 0.511 MeV photons. That interaction represents a conversion of mass to radiant electromagnetic energy.
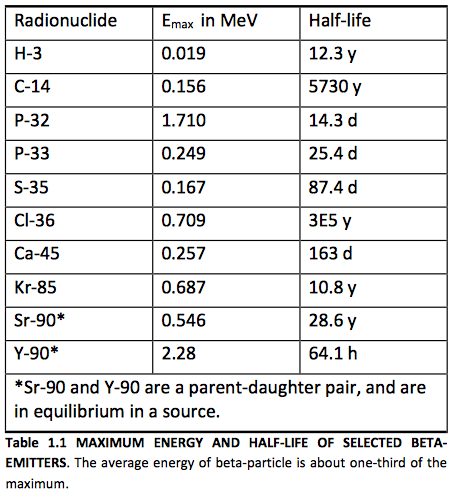
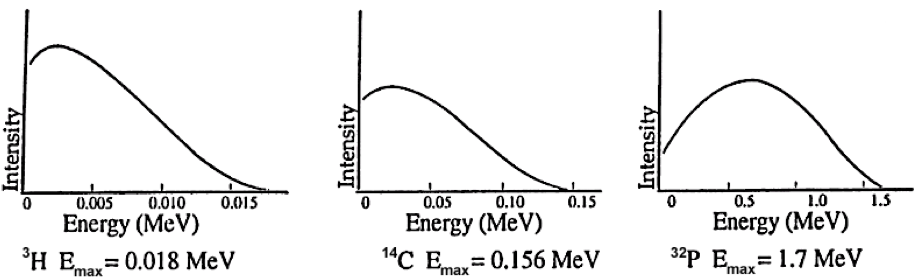
FIGURE 1.1 TYPICAL BETA‐SPECTRA. Beta spectra demonstrate two characteristics: maximum beta particle energy; the average beta particle energy (typically about one‐third of the maximum).
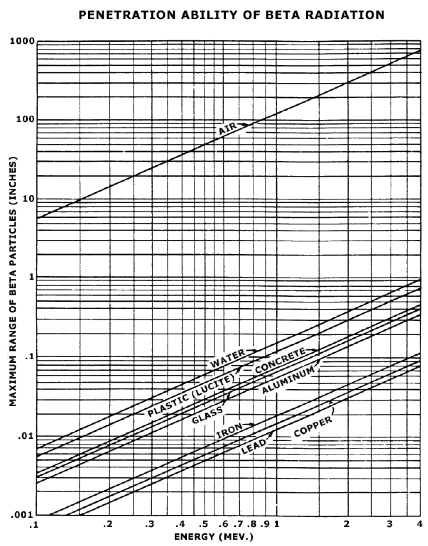
FIGURE 1.2 MAXIMUM RANGE OF BETA‐PARTICLES AS A FUNCTION OF ENERGY IN THE VARIOUS MATERIALS INDICATED. From Radiological Health Handbook, p. 122.
Beta‐Gamma
Most beta emitters decay to an excited daughter state that releases excess energy from the nucleus as a gamma ray. A gamma ray is simply a high energy photon emitted by a nucleus during its transition from a higher energy excited state to a lower energy unexcited state. Gamma rays are always preceded by a charged particle decay, most commonly a beta‐event. For example,
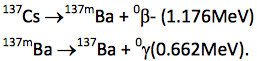
Although the second decay, called an isomeric transition from the metastable state to the ground state, has a half‐life of 2.54 minutes, we seldom chemically separate the 137mBa daughter from the 137Cs parent. Thus, it is not uncommon to colloquially refer to a “.662 MeV cesium‐137 gamma ray,” although it in fact emanates from a metastable barium nucleus. See Krane ch. 10.
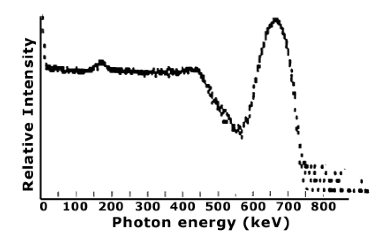
FIGURE 1.3 TYPICAL GAMMA RAY SPECTRUM. The spectrum of gamma rays emitted by a given isotope have distinct, characteristic energy peaks that permit identification of the isotope. This is Cs‐137 spectrum taken with a NaI (TI) detector.
Isomeric transition
If a metastable daughter is sufficiently long‐lived, it can be chemically separated from the parent, thus yielding a pure gamma emitter. The most important example is
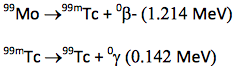
The half‐lives of the reactions are 2.7 days and 6.0 hours respectively. Thus it is possible to chemically separate 99mTc from its parent sample of 99Mo, yielding a pure gamma emitter sample with a half‐life of 6.0 hours. Tc‐99m is the radionuclide of choice for non‐invasive nuclear medicine imaging.
Internal conversion
If an excited, metastable nucleus goes to its ground state by transferring its energy to a valence electron that is ejected, the process is called internal conversion. This is observed more frequently in heavy nuclei; gamma decay is the preferred mode for lighter nuclei.
Electron capture
Some proton‐rich radionuclides decay by electron capture. An orbiting electron, usually from the K‐shell, enters the nucleus and combines with a proton to yield a neutron. Its vacancy is filled by a cascading valence electron, which releases its excess energy as a characteristic x‐ray. Alternatively, the excess energy can cause the ejection of a valence electron, called an Auger electron.
Spontaneous fission
A few very massive nuclei, such as Cf‐252, can decay by spontaneous fission. About 97% of Cf‐252 atoms decay by alpha emission. The remaining 3% of the neutron‐rich nuclei split into two lighter nuclei, with the release of an average 3.8 neutrons per fission event.
Neutrons
Small neutron sources can be fabricated by mixing an alpha‐emitter such as 238Pu or 241Am with 9Be, which has a loosely bound neutron. The nuclear reaction is:
![]()
These sources are commonly used in physics and analytical chemistry experiments when a low‐flux neutron source is needed.
