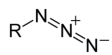
Azides are energy-rich molecules with many applications. Sodium azide, for example, is used as a preservative, mutagen, biocide, and assay reagent. Organic azides are capable of a great diversity of organic reactions and are important components in the azide-alkyne “click” reaction.
What are the hazards?
Caution should be exercised when using azides. Both organic and inorganic azides can be heat- and shock-sensitive and can explosively decompose with little input of external energy.
Caution should be exercised when using azides. Both organic and inorganic azides can be heat- and shock-sensitive and can explosively decompose with little input of external energy.
- Exposure to small amounts of sodium azide can result in rapid breathing, restlessness, dizziness, weakness, headache, nausea, vomiting, rapid heart rate, red eyes, clear drainage from the nose, coughing, skin burns, and blisters.
- Do not use halogenated solvents (such as methylene chloride or chloroform) for sodium azide reactions, as this can result in the formation of potentially explosive diazidomethane and triazidomethane, respectively.1
- Do not concentrate azide-containing reaction mixtures through rotary evaporation or distillation.
- Do not use metal spatulas for weighing and transferring azides.
- Do not expose organic azides to ground glass joints as this may cause the azide to decompose explosively.
How can I protect myself?
Planning for Use
- Write a Standard Operating Procedure for your experiment and review it with your principal investigator, using it as an opportunity to fully evaluate the hazards associated with your procedure and the materials you will be working with. Based on this evaluation, it may be determined that some of the safety precautions in this section are not applicable.
- Seek prior approval from your principal investigator if you plan to scale up the experiment beyond what was initially discussed with your PI, or if you plan to increase the amount of azide stored in the lab.
- Conduct dry runs to eliminate safety problems that may arise before azides are actually used.
- Use the smallest amount of azide possible for your experiment.
Controls
- Personal protective equipment must be worn, including a lab coat, safety glasses, and gloves with adequate chemical resistance.
- Conduct the experiment behind a blast shield in a fumehood with the sash positioned as low as possible. If use of a blast shield is not feasible, use a face shield.
- Keep the hood clear of any unnecessary chemicals and equipment. Clearly label your containers, and post a sign on the fumehood as notification that there is an azide experiment in progress.
- These precautions should be used for the whole duration of the experiment, including set up, work up, and clean up.
Conditions to Avoid
- Do not work alone.
How do I store this?
- Store synthesized azides below room temperature and away from sources of heat, light, pressure, and shock.
- Azides are generally classified as Storage Group X in the Stanford Storage Group Classification system, and should be stored away from all other chemicals. Specific incompatibilities include carbon disulfide, bromine, dimethyl sulfate, nitric acid, and heavy metals and their salts.
- Heavy metal azide salts tend to be highly heatand shock-sensitive explosives.
- Avoid water and strong acids which can lead to the formation of hydrazoic acid, which is highly toxic, volatile, and explosive.
How do I dispose of this?
Store azide waste in a container designated only for azide waste.
Dosimetry and bioassay requirements
Organic Azide Stability
Each chemical you intend to work with must be individually evaluated. Below are some general guidelines to consider when working with organic azides.
Carbon to Nitrogen Ratio
The total number of nitrogen atoms in your organic azide should not exceed that of carbon. Use the following equation to help evaluate if your azide is stable enough to work with, with N equal to the number of atoms: 1
(NCarbon + NOxygen) / NNitrogen ≥ 3
- n-nonyl azide (C/N ratio = 3) is the smallest organic azide that can be isolated and stored in its pure form (up to 20 grams).2
- Azides with a C/N ratio between 1 and 3 can be synthesized and isolated, but should be stored below room temperature at no more than 1 M concentration and at a maximum of 5 grams of material.2
- Organic azides with C/N ratio < 1 should never be isolated. It may be synthesized if the azide is a transient intermediate species AND the limiting reagent in the reaction mixture AND is limited to a maximum quantity of 1 gram.2
Rule of Six
Alternatively, follow the “rule of six:” six carbons (or other atoms of about the same size) per energetic functional group (azide, diazo, nitro, etc.) should provide enough dilution to render the compound relatively safe to work with given appropriate controls and safety procedures.3
In general, olefinic, aromatic, or carbonyl azides are much less stable than aliphatic azides.3
Reference
1Bräse, S., Gil, C., Knepper, K., Zimmermann, V. “Organic Azides: An Exploding Diversity of a Unique Class of Compounds,” Angew. Chem. Int. Ed., 2005, 44, 5188-5240.
2University of California Santa Barbara. “Laboratory Safety Fact Sheet #26: Synthesizing, Purifying, and Handling Organic Azides”
3Kolb, H.C., Finn, M.G., Sharpless, K.B. “Click Chemistry: Diverse Chemical Function from a Few Good Reactions,” Angew. Chem. Int. Ed., 2001, 40, 2004-2021.
