Protocols involving the use of rDNA/sNA for gene transfer into humans, whether done directly in the subject or in vitro and subsequently put into the subject, must be submitted to both the APB and the Stanford University Institutional Review Board (IRB) for Medical Human Subjects. Federal regulations require the local IBC (at Stanford, the APB), upon receiving submission of a Human Gene Transfer protocol, to review the following aspects to determine if NIH Recombinant Advisory Committee (RAC) review is required:
- The protocol uses a new vector, genetic material, or delivery methodology that represents a first-in- human experience, thus presenting an unknown risk.
- The protocol relies on preclinical safety data that were obtained using a new preclinical model system of unknown and unconfirmed value.
- The proposed vector, gene construct, or method of delivery is associated with possible toxicities that are not widely known and that may render it difficult for oversight bodies to evaluate the protocol rigorously.
Dependent upon the above findings the protocol will be either be submitted for RAC review or the APB will state that RAC review is not required.
For additional information concerning Stanford University’s IRB panels, please access the panel’s web site at https://researchcompliance.stanford.edu/.
Table 1. Recombinant and Synthetic Nucleic Acid Molecules (NIH guidelines).
If your experiment is in an exempt category, APB approval is not necessary. If your experiment does not fall within the exempt categories, you must have current APB approval (also see Table 2).
| Is your synthetic nucleic acid designed to: (1) neither replicate nor generate nucleic acids that can replicate in any living cell, and (2) not integrate into DNA, and (3) not produce a toxin that is lethal for vertebrates at an LD50 of less than 100 nanograms per kilogram body weight? | Yes | Exempt (III-F-1) |
| Is your recombinant or synthetic nucleic acid molecule not in an organism, cell or virus and not been modified or manipulated to render it capable of penetrating cellular membranes? | Yes | Exempt (III-F-2) |
| Is your recombinant or synthetic nucleic acid molecule solely from a single source that exists contemporaneously in nature? | Yes | Exempt (III-F-3) |
| Is your recombinant or synthetic nucleic acid molecule solely from a prokaryotic host and propagated in the same host or transferred to another host by naturally occurring means? | Yes | Exempt (III-F-4) |
| Is your recombinant or synthetic nucleic acid molecule from a eukaryotic host and propagated in the same host? | Yes | Exempt (III-F-5) |
| Is your recombinant or synthetic nucleic acid molecule from species that naturally exchange DNA? | Yes | Exempt (III-F-6) |
| Does your genomic DNA contains a transposable element that does not contain any recombinant and/or synthetic nucleic acids? | Yes | Exempt (III-F-7) |
| Recombinant or synthetic nucleic acid molecule which does not present a significant risk to health or the environment, as determined by the NIH* | Yes | Exempt (III-F-8) |
Table 2. Experiments requiring APB approval.
| Approval required for experiments involving: (Specific NIH Guideline Section) |
Further Information and Examples: |
|---|---|
| Deliberate transfer of a drug resistance trait to microorganisms that are not known to acquire the trait naturally if such acquisition could compromise the ability to control disease agents in humans, veterinary medicine or agriculture. (III-A-1-a) |
|
| Cloning of DNA, RNA or synthetic nucleic acid molecules encoding toxins lethal to vertebrates at an LD50 of <100 ng/kg body weight. (III-B-1) |
|
| Transfer of recombinant or synthetic nucleic acid molecules, or DNA or RNA derived from recombinant or synthetic nucleic acid molecules into human research participants. (III-C-1) |
|
| Risk Group 2, Risk Group 3, Risk Group 4 or Restricted Agents used as Host-Vector Systems. (III-D-1) |
|
| DNA from Risk Group 2, Risk Group 3, Risk Group 4 or Restricted Agents cloned into nonpathogenic prokaryotic or lower eukaryotic host-vector systems. (III-D-2) |
|
| Infectious DNA or RNA viruses or defective DNA or RNA viruses in the presence of helper virus in tissue culture systems. (III-D-3) |
|
| Whole animals, including transgenic animals. (III-D-4) |
|
| Whole plants. (III-D-5) |
|
| Large-scale DNA work. (III-D-6) |
|
| Influenza virus. (III-D-7) |
|
b Refers to ability of vector to infect cells from a range of species. Ecotropic generally means able to infect only cells of the species originally isolated from or identified in. Please note that the ecotropic host for HIV and HSV would be human cells, but the ecotropic host for MMLV would be murine cells. Amphotropic and VSV-G-pseudotyped virus host range includes human cells.
c Shown are general categories of cellular genes and functions. Please note that there are differences in the containment level for the same class depending on whether the viral vector integrates into the recipient genome at a high rate. The general categories are as follows: S, structural proteins (actin, myosin, etc.); E, enzymatic proteins (serum proteases, transferases, oxidases, phosphatases, etc.); M, metabolic enzymes (amino acid metabolism, nucleotide synthesis, etc.); G, cell growth, housekeeping; CC, cell cycle, cell division; DR, DNA replication, chromosome segregation, mitosis and meiosis; MP, membrane proteins, ion channels, G-coupled protein receptors, transporters, etc.; T, tracking genes such as those for green fluorescent proteins and luciferases and photoreactive genes; TX, active subunit genes for toxins such as ricin, botulinum toxin and Shiga and Shiga-like toxins; R, regulatory genes for transcription and cell activators such as cytokines, lymphokines and tumor suppressors; Ov and Oc, oncogenes identified via transforming potential of viral and cellular analogs, or mutations in tumor suppressor genes resulting in a protein that inhibits/moderates the normal cellular wild- type proteins. This does not include SV40 T antigen. SV40 T-antigen-containing cells should not be considered more hazardous than the intact virus. SV40 is considered a risk level 1 agent (the lowest level) according to the NIH Guidelines. The prevalence of SV40 infection in the U.S. population due to contaminated polio vaccine does not seem to have caused a statistically significant increase in the rate
of cancers. However, the data from the various studies on SV40 association with cancer are equivocal (Strickler et al. 1998; Butel and Lednicky, 1999; Dang-Tan et al., 2004).
d This is a general assessment of containment levels for laboratory construction and use of these vectors for nonproduction quantities only based on the 4th edition of BMBL. This table cannot cover every potential use within a research or laboratory settings; as information is gained, risk assessments and containment levels may be changed. Local IBCs should use all available information and their best judgment to determine appropriate containment levels. BSL – 1* refers to the containment level based on parent virus risk group. However, most procedures involving the handling and manipulation of the viral vectors are done at BSL – 2 to protect cell cultures and viral stocks from contamination.
e Certain specific strains of poxviruses, such as MVA, NYVAC, ALVAC and TROVAC, are considered low-risk agents and can be handled at BSL – 1 in certain cases.
From Biological Safety Principles and Practices, 4th ed., pg. 524, D.O. Fleming and D.L. Hunt, Ed, ASM Press, 2006.
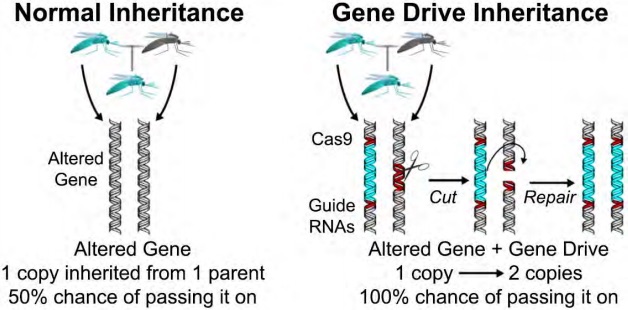
Image credit: Kevin Esvelt
![]() Important Information
Important Information
Human Gene Transfer
Conducting Gene Transfer experiments into human subjects requires both an IRB and an APB protocol.
