Peroxides can be formed via intentional chemical reactions (i.e., ozonolysis), but just as hazardous is inadvertent peroxide formation during storage of certain compounds. Some compounds form explosive peroxides. Others are polymerizable unsaturated compounds that can participate a runaway, explosive polymerization reaction catalyzed by peroxides. To varying degrees, shock, heat, or friction may cause unexpected explosion of peroxidized organic chemicals.
Common classes of compounds that form peroxides include:
- Ethers, acetals, and ketals, especially cyclic ethers and those with primary and/or secondary alkyl groups
- Aldehydes, including acetaldehyde and benzaldehyde
- Compounds containing benzylic hydrogens
- Compounds containing allylic hydrogens, including most alkenes; vinyl and vinylidene compounds, and dienes
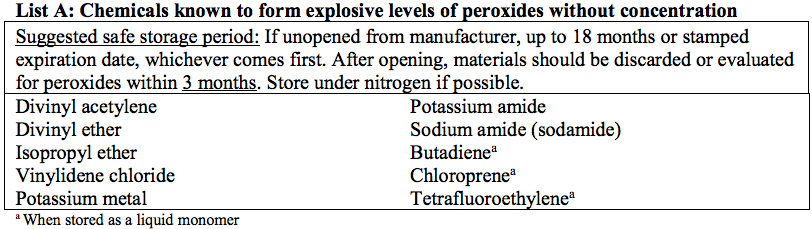
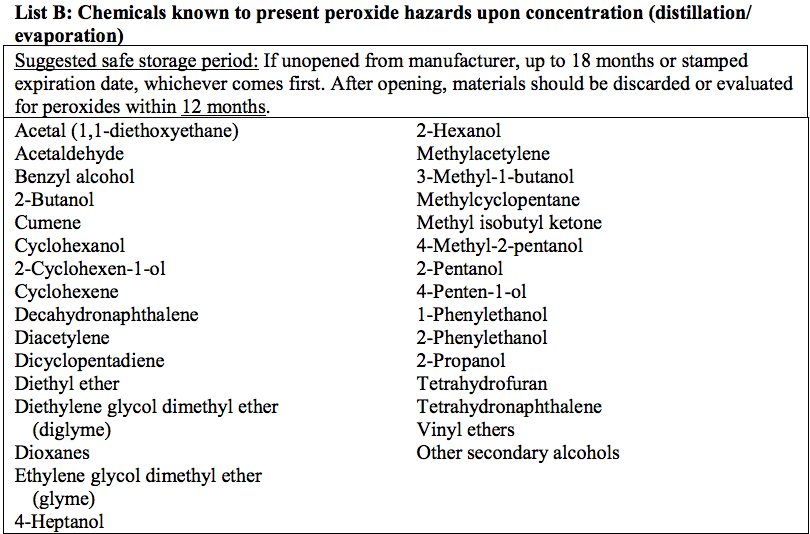
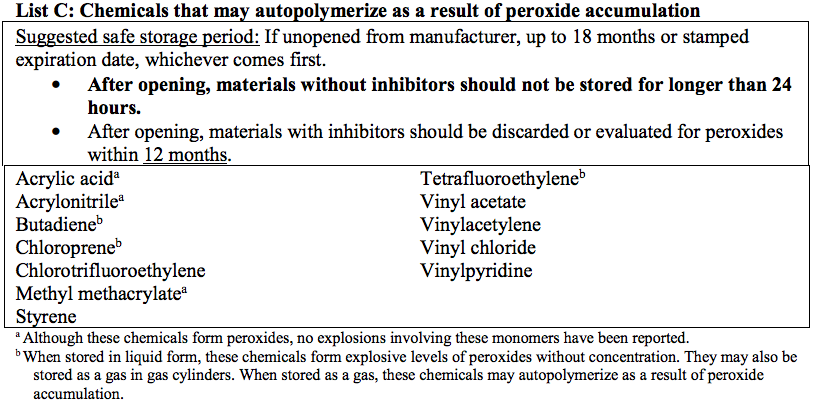
Refer to the Appendix for a list of common peroxide forming chemicals and recommendations on safe storage durations.
What are the hazards?
Chemical selection:
- If possible, use a material that does not form peroxides.
- If possible, purchase peroxide forming chemicals containing an inhibitor such as butylated hydroxytoluene (BHT).
How can I protect myself?
- Use fume hood or other appropriate exhaust ventilation if inhalation hazard is anticipated.
- Wear appropriate lab attire and personal protective equipment (PPE).
- Utilize shields, barricades, and additional PPE (such as face shields with throat protectors and heavy gloves) where there is a possibility of explosion or vigorous chemical reaction.
Evaluating Peroxide Formers:
Prior to using peroxide formers and as needed (see Appendix), conduct the two-part evaluation for peroxide content:
Part 1. Initial Screening—Verify:
- Identity of chemical
- Date last opened (or if unopened, date received) is known and is within the recommended safe storage period per guidance in Appendix A
- Evaporation of the chemical is known or estimated to be less than 10%
- Container shows no visible discoloration, liquid stratification, or crystallization (around the cap or in solution)
CAUTION: Never try to force open a rusted or stuck cap on a container of a peroxide-forming chemical.
If any items above cannot be verified, the container should be considered unsafe and should not be disturbed (promptly contact EH&S at 725-9999 for assistance with safe disposal).
Part 2. Peroxide Testing
Containers passing the initial screening (Part 1) may be tested for peroxide content. Four peroxide detection methods are commonly used. They include two qualitative variations on the iodine detection method, the qualitative ferrous thiocyanate method, and the use of semi-quantitative redox dip strips.
Dip strips provide the highest sensitivity and the most accurate quantification of peroxide concentration for routine testing. They are easier, faster and safer to use than other methods and detect a wider range of peroxides. However, dip strips are inconvenient to use for testing nonvolatile solvents and have a limited shelf life.
For inorganic peroxides such as hydrogen peroxide: the MQuant™ Peroxide Test Strip (0-100 ppm range). Available through Fisher Scientific, Catalog #M1100810001
For Organic Peroxides such as peracetic acid and hydroperoxides: The Quantofix test kits from Sigma Aldrich Catalog #Z101680
Caution: Read all instructions regarding interference and other conditions. Test strips must be stored in accordance with manufacturer recommendations.
Assessing Peroxide Levels:

How do I store this?
- The quantity of peroxide-forming chemicals kept should be restricted to the minimum amount needed.
- Store peroxide-formers in airtight bottles, away from light and heat. Avoid using containers with loose-fitting lids and ground glass stoppers.
- Certain peroxide formers, including those in Appendix A List A, should be stored under nitrogen if possible.
- Evaluate chemicals for peroxide formation regularly and always prior to distillation (see “Evaluating Peroxide Formers”). Some materials may need evaluation as often as every 3 months.
- Crystallization, discoloration, and stratification are signs a peroxide former may have become shock sensitive – Do not move the container, and call EH&S promptly at 725-9999.
- If evaporation or distillation is necessary, do not distill to a dry residue. Always leave at least 10-20% residual bottoms.
Labeling
All peroxide-forming compounds should be labeled as shown to the left, with the date received and the date opened. For common peroxide formers, guidance on recommended shelf life and time interval for peroxide evaluation is provided in the appendix.
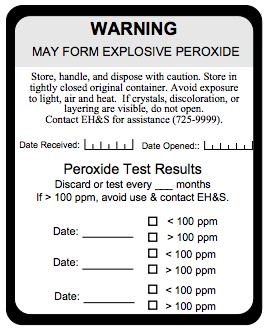
Contact EH&S at (650) 723-0448 for labels.
Appendix
Lists of Common Peroxide Forming Chemicals
(NOTE: The lists below cover many commonly known peroxide formers, but are not all-inclusive)
Reference
National Research Council, Prudent Practices in the Laboratory, National Academy Press: Washington, DC, 1995.
Kelly , R.J. “Review of Safety Guidelines for Peroxidizable Organic Chemicals,” Chemical Health & Safety- American Chemical Society–, 1996, 4(5), 28-36.
