General Decontamination Procedure (Figure 4)
- Put on proper PPE
- Place an absorbent material (paper towel, bench diaper) over the contaminated surface, then add liquid disinfectant; this will prevent spread of contamination.
- Allow sufficient contact time after applying the disinfectant. If the contact time is too brief, the surface will not be thoroughly disinfected.
- When cleaning a spill of concentrated material or if the disinfectant must act on an uneven surface, allow extra time for the disinfectant to act.
- Avoid using concentrated or undiluted solutions of your disinfectant to “speed up” the inactivation process. The surface that is being disinfected may be adversely affected by strong chemicals. This is especially significant when working with bleach, which is a very strong corrosive. Some disinfectants will leave a residue of chemicals behind.
- Rinse the cleaned area with distilled water to avoid adverse effects on your experiment. This is especially important in tissue culture rooms where a cell line can be wiped out by disinfectant residue left on equipment.
Disinfectant Selection
Disinfectant selection is based on several factors (Figure 5):
- What is the target organism that you wish to inactivate?
- What are the physical characteristics of the surface which will be disinfected? (porous surfaces may absorb disinfectants; some disinfectants may corrode metal surfaces).
- How long will the contact time be between the disinfectant and the target organism? (high concentrations of biological organisms may require longer contact times).
Note that the disinfection of prions and prion-like proteins must follow specific guidelines.
It is important to note that ‘bleach’, a very common and effective disinfectant, is not stable at dilute concentrations; working dilutions of sodium hypochlorite should be made weekly from a stock solution. Working solutions of 10% bleach (1:10 dilution of household bleach in water) is effective in most situations. Note that undiluted bleach must not go down the drain.
Alcohol based disinfectants will also evaporate over time and should be made up at appropriate intervals.
The following list of disinfectants, their efficiencies, contact times and recommended dilutions are general guidelines—please follow specific manufacturer’s recommendations if available.
Quaternary Ammonium Compounds are commonly used in floor cleaning solutions. Quaternary ammonium compounds are effective in inactivating most vegetative bacteria, fungi, and lipid containing viruses. Quaternary ammonium compounds are NOT effective when used to disinfect Mycobacterium tuberculosis (TB), bacterial spores, and many viruses such as HBV.
- Recommended contact time: 10 minutes
- Recommended Working Dilution: 0.1-2.0%
- Recommended for: cleaning optical instruments and administrative areas in the vicinity of a laboratory
Ethanol is commonly used on equipment whose surfaces are susceptible for corrosion if other disinfectants are applied. Ethyl alcohol is effective in inactivating most vegetative bacteria, fungi, and lipid containing viruses. Ethanol is NOT effective when used to disinfect HBV, Mycobacterium tuberculosis (TB) and bacterial spores.
- Recommended contact time: 10 minutes
- Recommended Working Dilution: 70-85%
- Recommended for: Stainless steel surfaces. CAUTION: Do not use 70% ethanol to clean a Class II, type A recirculating biosafety cabinet. The vapors from ethanol are flammable and the lower explosive limit (LEL) for ethanol is easily attained
Phenolics are commonly used to decontaminate surfaces such as lab bench tops. Phenolics are effective in inactivating vegetative bacteria, fungi, TB, lipid containing viruses and have some effect on HBV. However, phenolics will not inactivate bacterial spores.
- Recommended contact time: 10 minutes
- Recommended Working Dilution: 1.0-5.0%
- Recommended for: an alternative to bleach as a broad-spectrum disinfectant for bench tops, floors, and metal surfaces. Phenolics will not corrode metal surfaces as readily as bleach
Iodine-containing compounds or iodophors are commonly used to decontaminate metal surfaces or equipment. Iodophors are effective in inactivating vegetative bacteria, fungi, TB and lipid containing viruses and have some effect on HBV. However, iodophors will not inactivate bacterial spores.
- Recommended contact time: 10 minutes
- Recommended Working Dilution: 25-1600 ppm, 0.47%
- Recommended for: biosafety cabinets, dental equipment, bench tops, floors and lab equipment in general
Chlorine compounds such as bleach are commonly used in the lab because of the relative ease in accessibility and low cost. Chlorine (hypochlorite) compounds are effective in inactivating vegetative bacteria, fungi, lipid and non-lipid viruses, Coxiella burnetii and TB. Chlorine compounds have some effect in inactivating bacterial spores.
- Recommended contact time: 30 minutes
- Recommended Working Dilution: 500 ppm (1:10 dilution of household bleach, 5% hypochlorite ion)
- Recommended for: floors, spills (inactivating liquid specimens), bench tops and contaminated clothing. Do not use bleach on electronic equipment, optical equipment or unpainted stainless steel. Undiluted bleach and other disinfectants must not go down the drain
Paraformaldehyde and formaldehyde are often used to decontaminate large pieces of laboratory equipment, such as biosafety cabinets (but only by professionals!). Paraformaldehyde/formaldehyde will inactivate vegetative bacteria, fungi, lipid and non-lipid viruses, HBV, TB, Coxiella burnetii, and bacterial spores. However, paraformaldehyde and formaldehyde are registered carcinogens in the State of California and are very toxic to use without the accessibility of a vented fume hood and/or personal protective equipment. Do not use paraformaldehyde or formaldehyde in the lab to decontaminate equipment. The approved biosafety cabinet contractor will use paraformaldehyde to decontaminate your biosafety cabinet prior to changing the HEPA filters. Be sure to avoid using the biosafety cabinet while this operation is in effect!
Glutaraldehyde is often used to disinfect hospital instruments. Glutaraldehyde will inactivate vegetative bacteria, fungi, lipid and non-lipid viruses, HBV, TB, Coxiella burnetii, and bacterial spores. However, glutaraldehyde is very toxic to use without the accessibility of a vented fume hood and/or personal protective equipment. Do not use glutaraldehyde in the lab to decontaminate equipment.
Ethylene Oxide is often used to disinfect hospital instruments. Ethylene oxide will inactivate vegetative bacteria, fungi, lipid and non-lipid viruses, HBV, TB, Coxiella burnetii, and bacterial spores. However, Ethylene oxide is a registered carcinogen in the State of California and is very toxic to use without mechanically generated ventilation exhaust and personal protective equipment. Do not use ethylene oxide in the lab to decontaminate equipment.
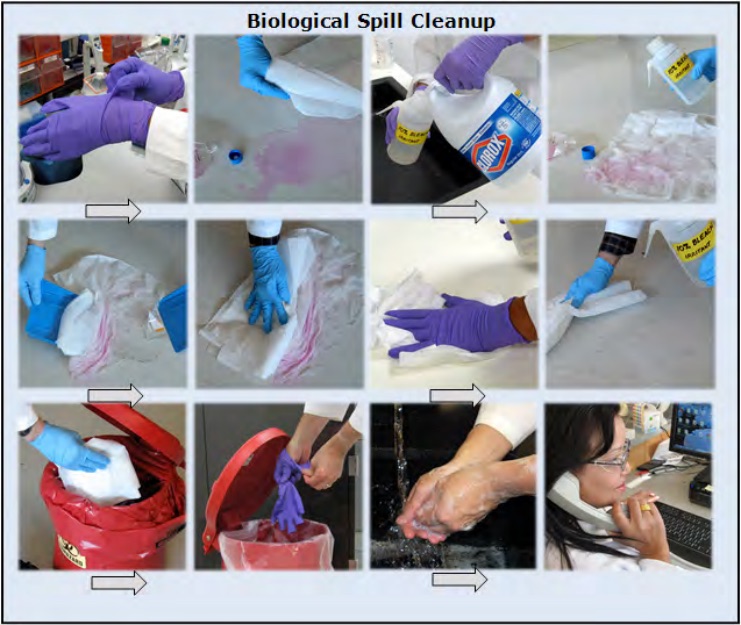
Choose disinfectant based on biological present in spill.
Length of time required for contact depends upon biological and disinfectant.
Dispose of materials as medical waste.
Report incident as required
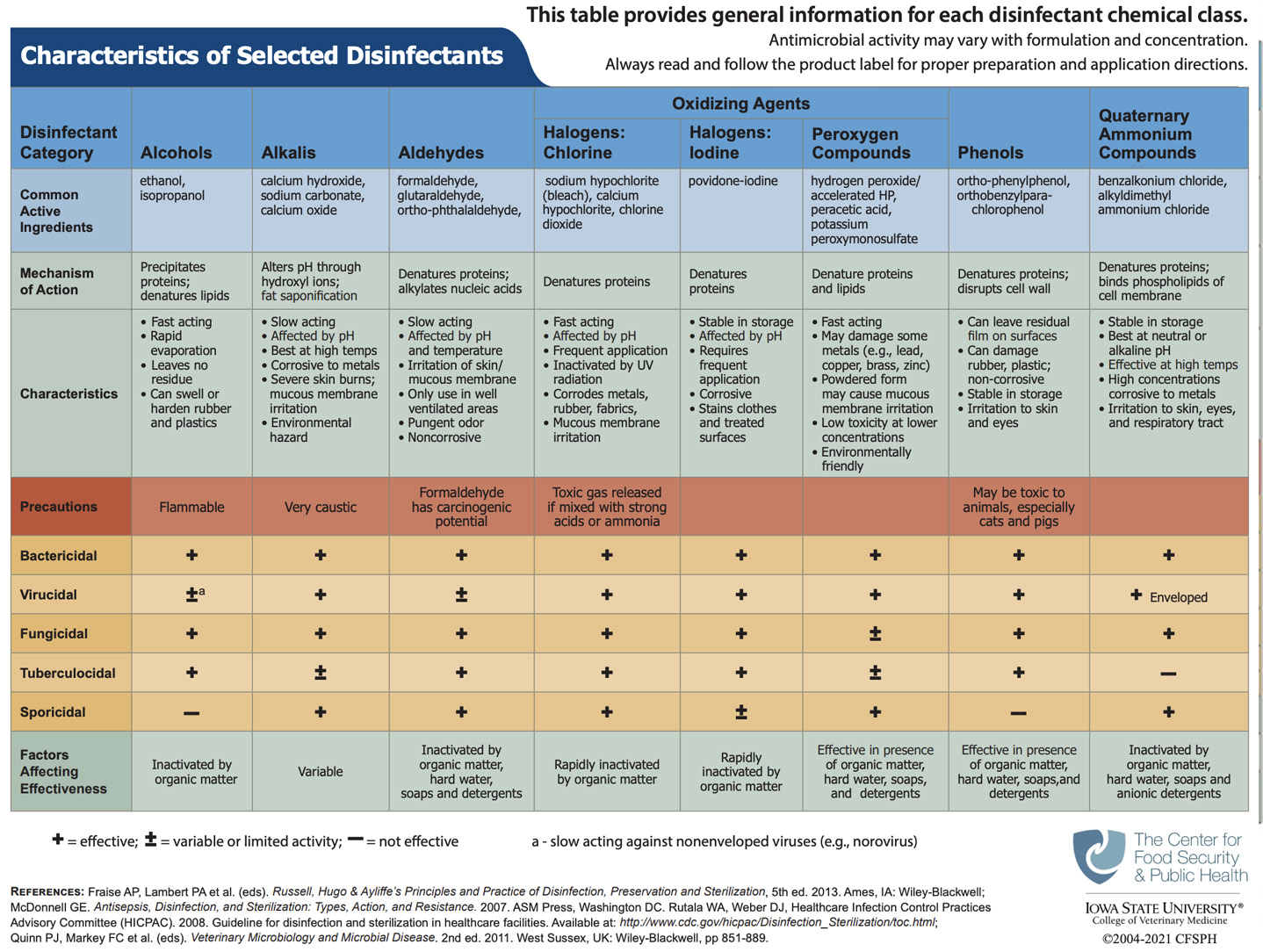
Courtesy of http://www.cfsph.iastate.edu/BRM/resources/Disinfectants/CharacteristicsSelectedDisinfectants.pdf
