Production of x‐ rays
Radioactivity is not the only source of ionizing radiation. Electrons are emitted by a filament heated with an electric current; the process is called thermionic emission. If the electrons are then accelerated through an electric potential of several kV to several MV, and then stopped instantly in a high atomic number metal target anode, some of their kinetic energy can be converted to high energy photons called bremsstrahlung radiation, from the German term for braking radiation. This radiation is more commonly known as x‐rays. However, most of the kinetic energy is converted to heat.
For electrons incident on a thick target, the fraction F of energy converted to x‐ rays is approximately:
F=7×10‐4 Z Ek
Z is the atomic number of the target, and Ek is the accelerating voltage in MV. Therefore, a 1 MV electron beam accelerated to a tungsten
(Z = 74) target will be about 5% efficient in the production of x‐rays.
F=7×10‐4 x74x1=0.052
The other 95% of the kinetic energy of the electrons is converted to heat.
Because x‐ray production is directly proportional to the atomic number of the target and the accelerating voltage of the device, reducing both variables can dramatically reduce the x‐ray output of a device. This explains why electron microscopes, cathode ray tubes, and television tubes are not significant sources of x‐ray exposure. Although they also have a heated filament and a beam of accelerated electrons, the target is a low Z material, and accelerating voltages are typically 20 kV to 50 kV. Because the maximum x‐ray energy cannot exceed the accelerating voltage, most of the x‐rays produced cannot penetrate the glass envelope used to contain the vacuum.
X‐ray spectra
An x‐ray spectrum is continuous, with energies ranging from near 0 keV to the maximum applied voltage. Intensity spikes at energies that are characteristic of the metal used to make the target are superimposed. See Figure 1.3. This process forms the basis for radiographic internal imaging in medicine. It is also used extensively in crystallography studies.
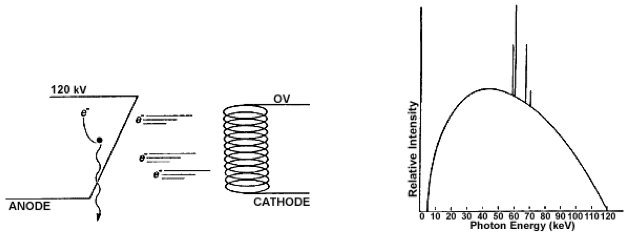
FIGURE 1.4 X‐RAYS. (a) x‐rays are produced when an electron loses kinetic energy while interacting with a target nucleus. (b) x‐ rays demonstrate a continuous bremsstrahlung spectrum with spikes that are characteristic of the anode target material, in this case tungsten. The maximum x‐ray energy, when expressed in keV, is equal to the voltage applied between the cathode and anode, in this case 120 kV. The average x‐ray energy is about one‐third of the maximum.
X‐ray diffraction and x‐ray fluorescence
A special word of caution is appropriate for those who use analytical x‐ray devices. Although the beam is narrow, its intensity can be 500 rads per second at the sample, and 10,000 rads per second at the x‐ray tube window. Just a few minutes handling a sample with the beam on could cause ulceration that can only be treated by amputation.
The dose rate for your unit can be calculated:
X (rad/sec) = 50 x V (kV) xI (mA) x Ztarget / [r (cm)]2 x74
For example, the dose rate at 2 cm from a copper target operated at 80 kV and 100mA is:
50 x 80 x 100 x 29/ (2)2 x 74=39000rad/sec
For a further discussion, see Health Physics. 15(6):481‐486, December 1968.
Neutrons
Neutrons can be created by bombarding targets with high energy photons:
γ + 9Be → 8Be + 1n,
or accelerated charged particles, for example deuterons:
2d+ 3He→4He+1n
